Advisory - Strawberry-flavoured acetaminophen infant oral drops in 24 mL bottles recalled because of defective child-resistant safety caps Français
OTTAWA, Dec. 7, 2018 /CNW/ -
Issue
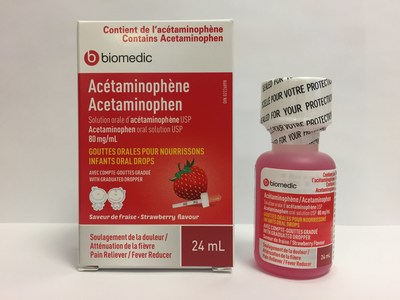
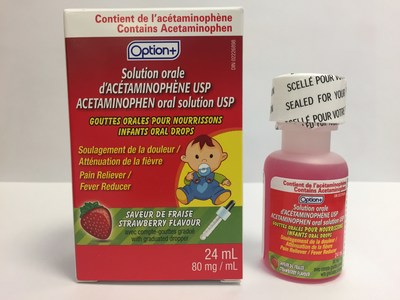
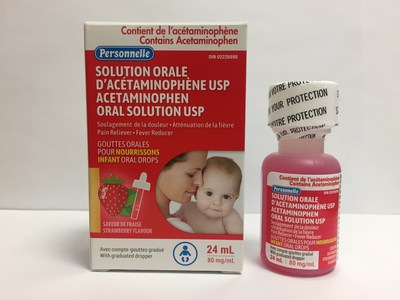
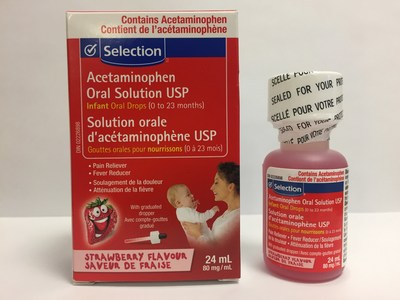
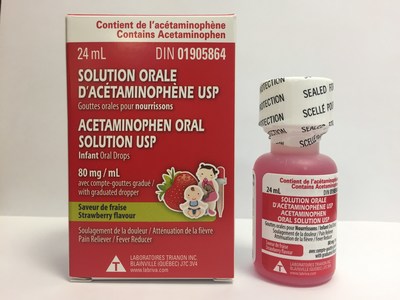
Laboratoire Riva Inc. and Laboratoires Trianon Inc. are voluntarily recalling five over-the-counter strawberry-flavoured acetaminophen oral drops for infants. The products are labeled as Biomedic, Option+, Personnelle, Selection, or Laboratoires Trianon Inc. The products are packaged in 24 mL bottles and are used for pain and fever relief. They are being recalled because the child-resistant safety cap may be defective. This recall is in addition to previous recalls of children's acetaminophen syrups for the same issue.
A defective cap could allow a child to accidentally ingest the product, which could pose a significant health concern. Accidental ingestion can result in acetaminophen overdose and serious health consequences, including liver damage. Early signs of overdose include nausea, vomiting, lethargy and sweating. Liver damage may result in liver failure or, in the most severe cases, death. Abdominal pain may be the first sign of liver damage and may not be apparent for 24 to 48 hours.
This issue is related to product packaging and not the safety or effectiveness of the drug in the bottles. The products were distributed at major pharmacies across Canada.
Who is affected
- Consumers who have bought the affected products listed in the table below
Affected products
Description |
Company |
DIN |
Lot |
Expiry |
Units Distributed |
Biomedic Acetaminophen (80 mg/mL) infant oral drops, strawberry flavour
24 mL bottle |
Laboratoire Riva Inc. |
02226898 |
C0217-3 |
20-FE |
2,016 |
C0217-4 |
20-FE |
1,056 |
|||
C0217-8 |
20-FE |
2,016 |
|||
C0735-5 |
20-JL |
4,224 |
|||
C0735-7 |
20-JL |
1,728 |
|||
F1213 |
18-DE |
4,128 |
|||
F1213-1 |
18-DE |
1,056 |
|||
Option+ Acetaminophen infant oral drops USP (80 mg/mL), strawberry flavour
24 mL bottle |
Laboratoire Riva Inc. |
02226898 |
C0217-2 |
20-FE |
2,016 |
C0217-5 |
20-FE |
1,056 |
|||
C0217-7 |
20-FE |
2,016 |
|||
C0217-A |
20-FE |
3,072 |
|||
C0217-B |
20-FE |
1,248 |
|||
F1213 |
18-DE |
6,624 |
|||
Personnelle Acetaminophen infant oral drops USP (80 mg/mL), strawberry flavour
24 mL bottle |
Laboratoire Riva Inc. |
02226898 |
C0735-1 |
20-JL |
2,598 |
C0735-4 |
20-JL |
2,592 |
|||
Selection Acetaminophen infant oral drops USP (80 mg/mL), strawberry flavour
24 mL bottle |
Laboratoire Riva Inc. |
02226898 |
C0735-2 |
20-JL |
2,397 |
Laboratoires Trianon Inc. Acetaminophen Oral solution USP (80 mg/mL) children's syrup, strawberry flavour
24 mL bottle |
Laboratoires Trianon Inc. |
01905864 |
C0217-1 |
20-FE |
1,056 |
C0217-6 |
20-FE |
1,098 |
|||
C0217-9 |
20-FE |
995 |
|||
C0735-3 |
20-JL |
1,536 |
|||
C0735-6 |
20-JL |
2,995 |
|||
F1213 |
18-DE |
4,602 |
|||
F1213-2 |
18-DE |
880 |
What consumers should do
- Return affected product to the place of purchase for a refund.
- Call your local poison control centre immediately if you think your child may have taken too much acetaminophen. Consult your health care professional if your child has used these products and has health concerns.
- Contact Urszula Wiatrowska, Pharmacovigilance Specialist at Laboratoire Riva and Laboratoires Trianon Inc., if you have questions about the recall. Email [email protected], or call toll-free at 1-800-363-7988, extension 236.
- Store medications out of reach and sight of children.
- Report any health product-related adverse reactions or complaints to Health Canada.
What Health Canada is doing
Health Canada is monitoring the recalls and is following up with the companies to confirm that appropriate actions are taken to prevent this situation from happening again. Should additional safety concerns be identified related to this issue, Health Canada will take appropriate action and inform Canadians as necessary.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709

Share this article