OTTAWA, Nov. 14, 2018 /CNW/ -
Issue
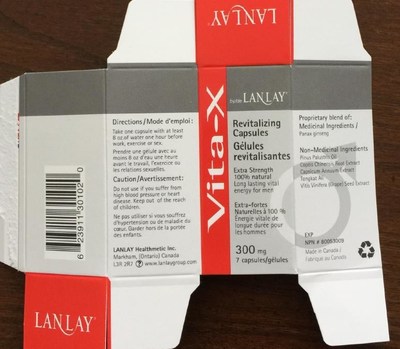
Health Canada is advising Canadians that two versions of "Vita-X Revitalizing Capsules" by Lanlay Healthmetic Inc., promoted for "long lasting vital energy for men," may pose serious health risks. One version contains seven capsules and has "NPN 80053009," a Natural Product Number (NPN) indicating Health Canada authorization, on the label. The second version contains one capsule, has no NPN on the label and is not authorized by Health Canada.
Health Canada tested the unauthorized version, which it had seized from an Etobicoke, ON, store, and found it to contain sildenafil, a prescription drug that was not declared on the label. Neither version of the product is authorized to contain sildenafil. Lanlay Healthmetic Inc. has confirmed that the two versions are manufactured by the same contract manufacturer, which means that it is possible that both versions contain sildenafil.
As a result, Health Canada has suspended the product licence (NPN), which means it is now illegal to sell this product in Canada. Health Canada has also asked the company to stop selling and to recall both versions of the product.
Who is affected
- Consumers who have bought or used these products.
Affected products
- Vita-X Revitalizing Capsules (7 capsules, labelled with NPN 80053009)
- Vita-X Revitalizing Capsules (1 capsule, no NPN on the label)
What consumers should do
- Stop using these products. Consult your health care professional if you have used these products and have health concerns.
- Report any health product adverse events or complaints to Health Canada.
Background
Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Stay connected with Health Canada and receive the latest advisories and product recalls.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709


Share this article