Advisory - Duchesnay Inc. recalls certain lots of PregVit and PregVit folic 5 prenatal and postpartum vitamin-mineral supplements because of a packaging error Français
OTTAWA, Nov. 15, 2019 /CNW/ - Health Canada is advising Canadians that Duchesnay Inc. is recalling certain lots of PregVit vitamin-mineral supplements (for prenatal and postpartum use) and PregVit folic 5 vitamin-mineral supplements (for prenatal use) because blister packages may contain all pink (morning) tablets instead of the correct combination of pink and blue (evening) tablets. The company estimates the issue affects a very small number of products in certain lots (see the table below) and not all products.
There are no quality, safety or effectiveness concerns with the tablets themselves but there is the potential that patients may not take the proper combination of pink and blue tablets if they have received products impacted by the packaging error.
The pink and the blue tablets do not contain the same active ingredients. Women taking all pink tablets will not receive the intended vitamin and mineral supplements. In particular, the pink tablets do not contain folic acid, which is important for the healthy growth of an unborn baby and helps to reduce some types of birth defects called neural tube defects.
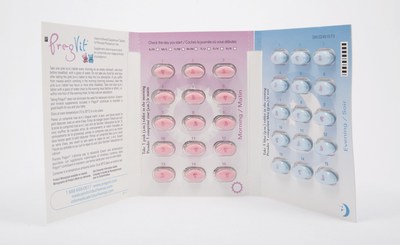
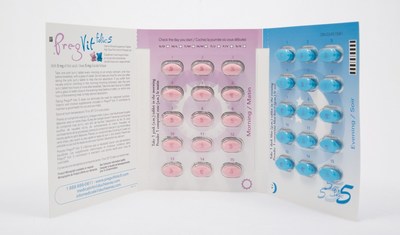
Properly packaged products should contain two blister packs, with each blister pack containing 15 pink tablets and 15 blue tablets. The company received a complaint of a product containing a blister pack that contained all pink tablets and no blue tablets.
Who is affected
Women who have bought or are using the affected products.
Affected products
Product |
DIN |
Lot |
Expiry |
PregVit Format 60 |
02451573 |
19003 |
2021-10-31 |
19001V |
2021-09-30 |
||
18015V |
2021-08-31 |
||
18010V |
2021-05-31 |
||
18012V |
2021-04-30 |
||
18006V |
2020-08-31 |
||
18003V |
2020-06-30 |
||
17019V |
2020-05-31 |
||
17016 |
2020-07-31 |
||
17002 |
2020-03-31 |
||
16065 |
2020-02-29 |
||
16064 |
2020-01-31 |
||
16045 |
2019-12-31 |
||
16042 |
2019-12-31 |
||
PregVit folic 5 Format 60 |
02451581 |
18022 |
2021-07-31 |
18007 |
2020-10-31 |
||
18002 |
2020-09-30 |
||
16053 |
2019-12-31 |
What consumers should do
- Check your product to make sure it contains the correct combination of pink and blue tablets (each blister pack should contain 15 pink and 15 blue tablets [see photos]).
- Continue taking the prescribed number of pink and blue tablets per day as directed by your health care professional. If your product has an incorrect tablet combination, talk to your pharmacist about obtaining a replacement. Properly packaged products do not need to be returned.
- Consult your health care professional if you have used either of these products and have health concerns.
- Contact Duchesnay Inc. by calling toll-free at 1-888-666-0611, or by email at [email protected], if you have any questions about the recall.
- Report any health product adverse events or complaints to Health Canada.
Additional information for pharmacists
- Check packages of PregVit and PregVit folic 5 before dispensing them to patients.
- Not all lots are being recalled. While the company has put a verification process in place to make sure that products not impacted by this recall are properly packaged, checking products before dispensing provides an added safeguard.
What Health Canada is doing
Health Canada is monitoring the recall and verifying that the company has effectively addressed the packaging issue. Should additional safety concerns be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709



Share this article