Advisory - Multiple unauthorized prescription drugs seized from Vanier store in Ottawa, Ontario, may pose serious health risks Français
OTTAWA, April 25, 2019 /CNW/ - Health Canada has seized multiple unauthorized drugs from Gigi's Market in Ottawa, Ontario (23 Montreal Road). The products are labelled to contain prescription drugs, including antibiotics, and may pose serious health risks. In addition, Health Canada seized two skin ointments that may contain a prescription-strength drug.
Prescription drugs should be taken only under the advice and supervision of a healthcare professional because they are used to treat specific diseases and may cause serious side effects.

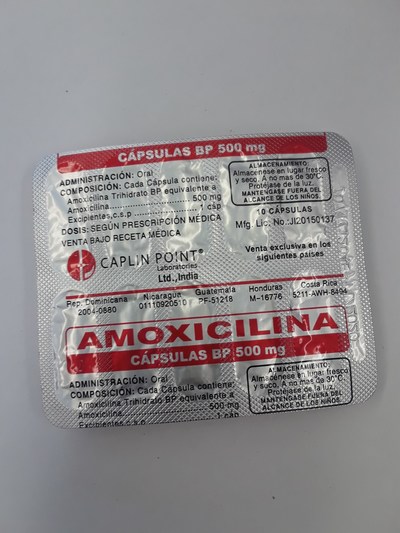

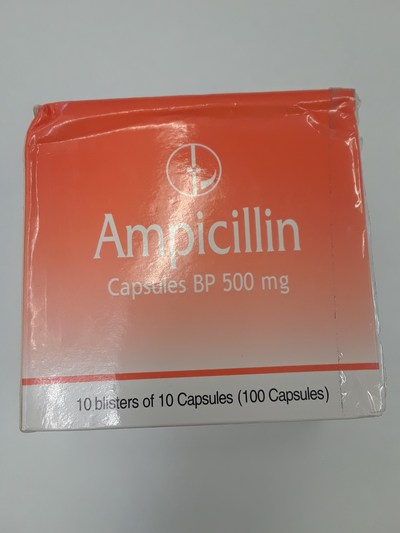




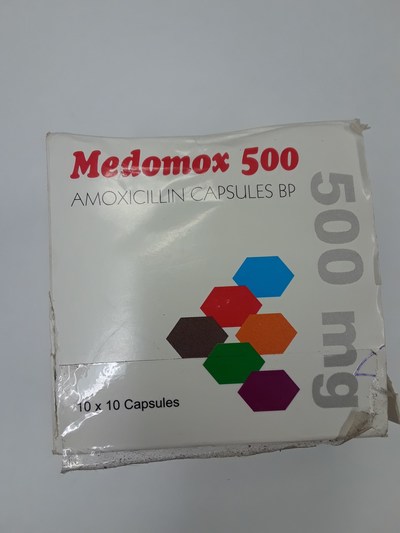

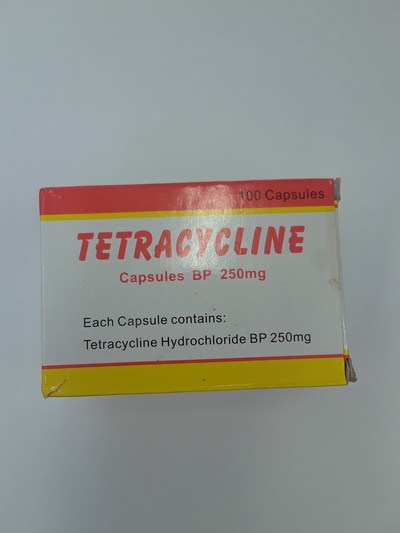


Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality and may pose serious health risks. They may contain ingredients, additives or contaminated ingredients not listed on the label. In addition, they may lack the active ingredients Canadians would expect them to contain to help maintain and improve their health or they may contain ingredients that could interact with other medications and foods. For all of these reasons, unauthorized health products could cause serious health effects. Selling unauthorized health products in Canada is illegal.
Some of the unauthorized health products are packaged and labelled in Spanish. As a result, information about ingredients, usage, dosage and side effects may not be understood by all consumers.
Who is affected
- Consumers who have bought or used any of the products shown below.
Affected products
Products that are labelled to contain a prescription drug:
- Amoxicillin 500 mg
- Amoxicilina 500 mg (amoxicillin)
- Ampicilina 500 mg (ampicillin)
- Ampicillin 500 mg
- Ampidel-500 (ampicillin)
- Dermovate cream 25 g (clobetasol propionate 0.05%)
- Erix*-100 mg (sildenafil citrate)
- Kamox 500 (amoxicillin)
- Medomox 500 (amoxicillin)
- Metrol 500 (metronidazole)
- Tetracycline 250 mg
Products that may contain a prescription-strength drug:
- Rx Lotion Rien N'était Valliéres – brown bottle (salicylic acid – concentration not listed on the label)
- Rx Lotion Rien N'était Valliéres – white bottle (salicylic acid – concentration not listed on the label)
What consumers should do
- Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related adverse reactions or complaints to Health Canada.
What Health Canada is doing
Health Canada seized the products from the retail location. Should additional safety concerns be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Background
Amoxicillin and ampicillin are prescription antibiotic drugs used to treat certain infections caused by bacteria. They should not be used by people who are allergic to them. Serious side effects with ampicillin and amoxicillin include severe allergic reactions (swollen nose, eyes, throat, difficulty breathing and skin rash), nausea, vomiting, diarrhea, and signs of kidney and liver problems. Unnecessary use or misuse of any antibiotic can lead to a decrease in its effectiveness.
Clobetasol propionate is a highly potent topical (applied to the skin) corticosteroid prescription drug used to treat inflammatory skin conditions. It should be used only under the supervision of a health care professional. It should not be used by people who are allergic to it. Side effects include skin irritation, weakening or deterioration. Topical corticosteroids can be absorbed in sufficient amounts to produce adverse effects, including symptoms of adrenal suppression (low blood pressure, low blood sugar, weight loss, muscle pain, gastrointestinal problems, and severe fatigue) or Cushing's syndrome (high blood pressure, high blood sugar, weight gain, muscle weakness, bone loss, and severe fatigue) depending on how much has been absorbed. Clobetasol should not be used by pregnant or nursing women.
Metronidazole is a prescription antibiotic and antiprotozoal drug. It should not be used by people who are allergic to metronidazole, or who have nervous system disorders, blood disorders, or underactive thyroid or adrenal glands. It should also be avoided by pregnant or nursing women. Drinking alcohol while taking metronidazole can cause a severe reaction (facial flushing, vomiting and rapid heartbeat), and should be avoided. Metronidazole can cause seizures, loss of sensation in the extremities (e.g., hands and feet), confusion and dizziness. It can cause reactions when combined with other medications, including certain blood thinners, lithium, cyclosporine, and anti-seizure drugs. Unnecessary use or misuse of any antibiotic can lead to a decrease in its effectiveness.
Sildenafil is a prescription drug used to treat erectile dysfunction and should be used only under the supervision of a health care professional. It should not be used by people who are allergic to it. As well, it should not be used by individuals taking any kind of nitrate drug (e.g., nitroglycerine) as it can cause potentially life-threatening low blood pressure. Individuals with heart problems are at increased risk of cardiovascular side effects such as heart attack, stroke, chest pain, high blood pressure and abnormal heartbeat. Other possible side effects include headache, facial flushing, indigestion, dizziness, abnormal vision, and hearing loss.
Salicylic acid is a prescription drug when sold for topical uses at concentrations greater than 20% or with a certain level of acidity (pH less than 3.0) except when sold to be applied to warts, corns or calluses. It is also used to treat acne. It should not be used by people who are allergic to salicylic acid, by people with diabetes, poor circulation, loss of sensation in the extremities (e.g., hands and feet), or by children and teenagers with the flu or chicken pox (as it may increase the risk of Reye's syndrome). It should not be used by pregnant or nursing women unless the area of exposure and duration of therapy is limited. Some products containing salicylic acid should not be used in children younger than two years of age. Salicylic acid can cause serious allergic reactions (hives, itching, trouble breathing, and swelling of the face, lips, or tongue), and severe skin irritation (redness, burning, dryness, itching, and peeling). It can also cause salicylate toxicity, a serious condition with nausea, vomiting, dizziness, loss of hearing, ringing in the ears, diarrhea, confusion, rapid breathing, and drowsiness. Side effects are more likely to occur in children and in people with kidney or liver disease, and with prolonged use over large areas. Salicylic acid should not be used on infected areas, on moles, birthmarks, warts with hair growing from them, or warts on the face.
Tetracycline is a prescription antibiotic drug used to treat infections. It should not be used by people who are allergic to tetracycline, or who have severe kidney or liver disease. It should not be used during pregnancy, as it could cause teeth and bone changes in the fetus and serious liver damage in the mother. Tetracycline also passes into breast milk and may cause tooth discolouration in a nursing baby. It should not be used in children under 12 years of age, as it can affect bone and cause permanent tooth discolouration. Tetracycline can increase skin sensitivity to sunlight (or sunlamps) and cause more severe sunburn reactions. Taking tetracycline with other medications, including retinoid drugs (for acne), digoxin, blood thinners (e.g., warfarin), and strontium, could cause serious reactions. Tetracycline can also make birth control pills less effective. Unnecessary use or misuse of any antibiotic can lead to a decrease in its effectiveness.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709

Share this article