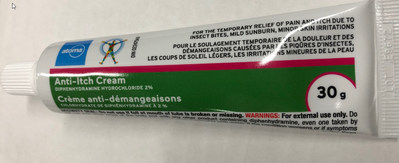
Advisory - One lot of Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream recalled because of a labelling error regarding use in children under two years of age Français
OTTAWA, Dec. 23, 2019 /CNW/ - Health Canada is advising Canadians, particularly parents and caregivers, that Taro Pharmaceuticals Inc. is recalling one lot of Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream because of a labelling error that may pose serious health risks to children under two years of age.
The text printed on the tube incorrectly states that, for children under two years of age, "Application should be supervised by an adult" when it should instead state "Consult a doctor."
There is no quality issue with the product, but the labelling error could lead to the inappropriate use of the product on children under two years of age. The company is recalling the mislabelled products from retail stores. Consumers are not being asked to return products.
Diphenhydramine hydrochloride in a 2% concentration cream is a non-prescription antihistamine drug used to relieve itching related to, for example, insect bites, sunburns, bee stings, poison ivy, poison oak and minor skin irritation. The drug may be absorbed into the skin in a significant amount, especially if the skin is broken. Diphenhydramine cream is not recommended for children under two years of age unless on the advice of a health care professional. Use of diphenhydramine hydrochloride in children under two years of age can increase the risk of side effects such as restlessness, irritability or agitation, trouble sleeping, and may include more serious side effects such as muscle spasms, trouble breathing and seizures.
Products from the affected lot have been sold at retail stores across Canada since July 29, 2019.
Who is affected
Parents and caregivers who have this product and who may use it on children under the age of two.
Affected products
Product |
DIN |
Lot number |
Expiry |
Atoma-brand Diphenhydramine Hydrochloride 2% Anti-Itch Cream, 30g |
02247560 |
E9672 |
April 30, 2022 |
What consumers should do
- Check if your product is affected by the labelling error. Consult a health care professional before using the affected product on children under two years of age.
- Consult a health care professional if you have used this product on a child under two years old and you have concerns about their health.
- Contact Taro Pharmaceuticals Inc. by email at [email protected] or by calling, toll-free, 1-800-268-1975, extension 5174 or 5138, if you have questions about this recall. Consumers are not being asked to return the product.
- Report any health product-related adverse reactions or complaints to Health Canada.
What Health Canada is doing
Health Canada is monitoring the company's recall and confirming that the company has taken the necessary steps to fix the labelling issue to prevent it from reoccurring. Should additional safety concerns be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709



Share this article