Advisory - Three unauthorized eye solutions seized from a Glorious Cosmetics store in Edmonton, Alberta, may pose serious health risks Français
OTTAWA, July 24, 2019 /CNW/ - Health Canada is advising consumers that it has seized three unauthorized eye health products—Digi Eye, Kobayashi Aibon/Eyebon Eyewash and Sante FX Neo―from a Glorious Cosmetics store in Edmonton, Alberta (10706, 82 Avenue NW) because they may pose serious health risks. According to the product labels, these products contain prescription drugs. Prescription drugs should be taken only under the advice and supervision of a healthcare professional because they are used to treat specific diseases and may cause serious side effects.
Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, effectiveness and quality and may pose serious health risks. They may contain ingredients, additives or contaminated ingredients not listed on the label. In addition, they may lack the active ingredients Canadians would expect them to contain to help maintain and improve their health or they may contain ingredients that could interact with other medications and foods. For all of these reasons, unauthorized health products could cause serious health effects. Selling unauthorized health products in Canada is illegal.
Some of the unauthorized health products are packaged and labelled in Japanese characters. As a result, information about ingredients, usage, dosage and side effects may not be understood by all consumers.
Who is affected
- Consumers who have bought or used any of the products shown below.
Affected products
Product |
Risk |
Digi Eye Eye drops |
Labelled (in Japanese) to contain neostigmine methylsulfate |
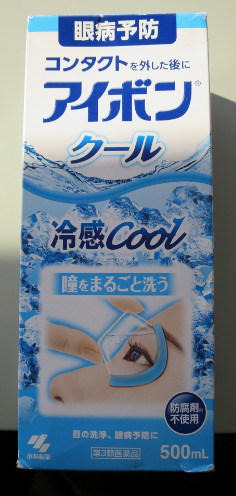
Kobayashi Aibon/Eyebon Eyewash Eyewash |
Labelled (in Japanese) to contain aminocaproic acid |
Sante FX Neo Eye drops |
Labelled (in English) to contain neostigmine methylsulfate and aminocaproic acid |
What consumers should do
- Stop using these products. Consult your health care professional if you have used any of these products and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related adverse reactions or complaints to Health Canada.
What Health Canada is doing
Health Canada is working with the Canada Border Services Agency to help prevent the importation of these products. Should additional safety concerns be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Background
Aminocaproic acid: This is a prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Neostigmine methylsulfate: There are no approved eye drops containing neostigmine methylsulfate on the Canadian market. In the past, drugs similar to neostigmine were used to treat glaucoma. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts, allergic reactions, iris cysts, retinal detachment and the potential for causing a specific type of glaucoma attack. In addition, absorption into the nose via the tear duct may cause serious cardiac and respiratory side effects.
SOURCE Health Canada

Media Enquiries: Également disponible en français, Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709



Share this article