Advisory - Unauthorized skin lightening product seized from Danforth Variety & Fruit Market: Product may pose serious health risks Français
OTTAWA, March 19, 2020 /CNW/ -
Summary
Product: Unauthorized skin lightening product from the Danforth Variety & Fruit Market in Toronto, ON.
Issue: The product is unauthorized and labelled to contain a prescription drug that may pose serious health risks.
What to do: Stop using this product. Consult your health care professional if you have used this product and have health concerns.
Issue
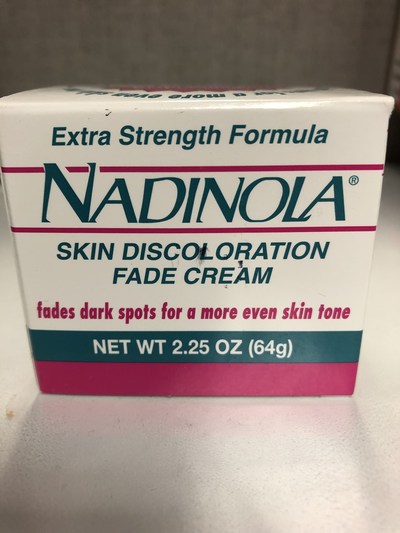
Health Canada is advising Canadians that it has seized an unauthorized prescription skin lightening product that may pose serious health risks from the Danforth Variety & Fruit Market (2742 Danforth Ave, Toronto, ON). The product is labelled to contain hydroquinone at a concentration of 3%, a prescription drug that may pose serious health risks, such as blisters, scarring, skin discolouration and possibly cancer.
Health Canada is encouraging Canadians to read the labels of products that claim to lighten, whiten, fade or bleach skin. Look for an eight-digit Natural Product Number (NPN) or Drug Identification Number (DIN) on the label, which indicates the product has been assessed by Health Canada for safety, efficacy and quality, and has been authorized for sale.
Health products that have not been authorized for sale by Health Canada may contain high-risk ingredients, such as prescription drugs, that may or may not be listed on the product label. Prescription drugs should be used only under the advice and supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects.
It is illegal to sell unauthorized health products in Canada. Health Canada has previously seized and warned Canadians about several unauthorized skin lightening health products whose labels show they contain prescription drugs (e.g., betamethasone dipropionate, clobetasol propionate, or hydroquinone at concentrations greater than 2%). The Department strongly encourages Canadians to not use these products and to report to Health Canada if they see the products for sale, so that the Department can take appropriate action.
Unauthorized skin lightening products may also contain unacceptable levels of mercury, a heavy metal. Mercury poisoning can cause serious harm, particularly to children, pregnant women and women who are breastfeeding. The Department has implemented measures to reduce the amount of heavy metals to which Canadians are exposed, including establishing strict limits for health products and cosmetics.
Who is affected
Consumers who have bought or who are using the affected product.
Affected product
Product |
Risk |
Nadinola Skin Discoloration Fade Cream (Extra Strength Formula) Skin lightening |
Product is labelled to contain 3% hydroquinone |
What consumers should do
- Stop using this product. Consult your health care professional if you have used this product and have health concerns.
- Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product adverse events or complaints to Health Canada.
What Health Canada is doing
Health Canada seized the product from the retail location after receiving a consumer complaint. Should additional retailers or distributors be identified, Health Canada will take appropriate action and inform Canadians as necessary.
Background
Hydroquinone for topical use at concentrations above 2% is a prescription drug used to lighten areas of darkened skin caused by different conditions (e.g., sun exposure, skin damage, pregnancy, medications or age). It should not be used by people who are allergic to hydroquinone or who are taking medicines that make their skin more sensitive to light. Hydroquinone is not recommended for pregnant or breastfeeding women, or children. It should be used with caution in those who have previously had cancer. Side effects include skin reactions such as redness, dryness, cracked skin, burning, stinging, peeling, itching, increased sensitivity to sunlight, sunburn, blisters and scarring. It may cause skin discolouration (i.e., blue or black discolouration or white patches or spots) that, in some cases, can be disfiguring. In laboratory animals, it has been associated with cancer after long-term exposure. As of June 30, 2019, products containing hydroquinone greater than 2% for topical use require a prescription from a healthcare practitioner to be sold in Canada. As part of this transition, several products exceeding 2% hydroquinone that were previously sold over the counter have been recalled in Canada.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866-225-0709, [email protected]






Share this article