TORONTO, March 8, 2018 /CNW/ -
AUDIENCE
Consumers, Physicians, Family Doctors, Gynaecologists, Obstetricians, Pharmacists and Nurses
KEY MESSAGES
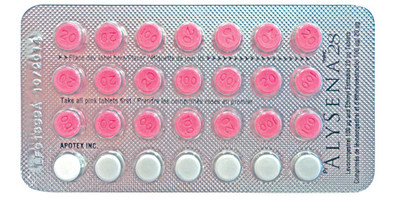
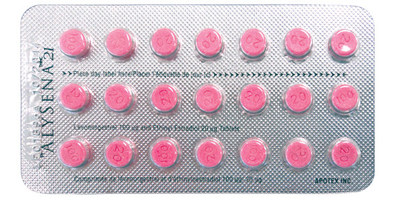
Apotex Inc. is advising consumers and health care professionals that complaints have been received for packages of ALYSENA™ 28 (21 active pills, and 7 inactive pills that contain no hormones) and ALYSENA™ 21 (21 active pills).
- Blister packages for ALYSENA™ 28 and ALYSENA™ 21 contain active (pink) pills that may be chipped.
- Taking pink pills that have a small chip at the edge will not reduce the product's overall effectiveness in preventing pregnancy.
- Health care professionals are advised to remind consumers to:
- Check pills before taking them and not consume pills that are chipped or look unusual (including jagged, broken or crumbling);
- Examine both sides of each pill thoroughly before taking it. It may not be immediately obvious from looking at the blister package that there is a problem with the pills, since the underside of the pill cannot be seen while in the blister pack.
- If a chipped pill is found, the next unchipped pink pill should be taken. Do not miss a dose.
- If you have no unchipped pink pills left, then return the package to your pharmacy as soon as possible for a replacement package. Use a non-hormonal method of birth control (such as condoms, spermicidal foam or gel) until a replacement package can be obtained and contact your health care provider for medical advice.
- Return blister packages containing chipped pills to the pharmacy to get a replacement package. Packages with no chipped pills do not need to be returned.
- Seek counsel on the proper use of oral contraceptives, and on what to do if you miss a dose as articulated in the patient information leaflet.
WHAT IS THE ISSUE?
Health Canada and Apotex Inc. have received reports for packages of ALYSENA™ 28 and ALYSENA™ 21 where the blister packages contained chipped active (pink) pill.
PRODUCTS AFFECTED
| Product |
DIN |
Strength |
Package / Format |
| ALYSENA™ 28 |
02387883 |
100 mcg / 20 mcg |
28 Tablets Blister Pack |
| Triple Pack 3x28 Tablets Blister Pack |
|||
| ALYSENA™ 21 |
02387875 |
100 mcg / 20 mcg |
21 Tablets Blister Pack |
| Triple Pack 3x21 Tablets Blister Pack |
BACKGROUND INFORMATION
ALYSENA™ is a prescription medicine used for:
1- Conception control.
2- Treatment of moderate acne vulgaris in women ≥14 years of age who have no known contraindications to oral contraceptive therapy, desire contraception, and have achieved menarche.
This product is manufactured by a 3rd party in Europe for Apotex Inc. During manufacturing, it is possible for pills to chip. However, the overall quality of the intact pills is not affected.
INFORMATION FOR CONSUMERS
ALYSENA™ is a birth control tablet (oral contraceptive) that contains two female sex hormones (levonorgestrel and ethinyl estradiol). Packages of ALYSENA™ 28 should have three rows of pink (active) pills and one row of white inactive (no hormones) pills. Packages of ALYSENA™ 21 should have three rows of pink (active) pills.
Patients are advised to:
- Check pills before and after taking them out of the blister package.
- Examine both sides of each pill thoroughly to determine if the pill is chipped. It may not be immediately obvious from looking at the blister package, as the underside of the pill cannot be seen while in the blister package;
- If a package has a chipped pill, return it to the pharmacy for a replacement package. Packages with no chipped pills do not need to be returned.
- Do not consume a pill if it's chipped or looks unusual (including jagged, broken or crumbling);
- Do not miss a dose of the birth control pill.
- If a chipped pill is found, the next unchipped pink pill should be taken. Do not miss a dose.
- If you have no unchipped pink pills left, then return the package to your pharmacy as soon as possible for a replacement package. Use a non-hormonal method of birth control (such as condoms, spermicidal foam or gel) until a replacement package can be obtained and contact your health care provider for medical advice.
Patients should not stop taking birth control pills unless advised to do so by their health care provider, as they could get pregnant. Patients should talk to their health care provider if they have questions or concerns about their birth control product, including missed doses and alternatives.
INFORMATION FOR HEALTH CARE PROFESSIONALS
Apotex Inc. advises health care professionals to remind their patients of the following:
- Check pills before and after taking them out of the blister package.
- Examine both sides of each pill thoroughly to determine if the pill is chipped. It may not be immediately obvious from looking at the blister package, as the underside of the pill cannot be seen while in the blister package;
- If a package has a chipped pill, return it to the pharmacy for a replacement package. Packages with no chipped pills do not need to be returned.
- Do not consume a pill if it's chipped.
- If a chipped pill is found, the next unchipped pink pill should be taken. Do not miss a dose.
- If you have no unchipped pink pills left, then return the package to your pharmacy as soon as possible for a replacement package. Use a non-hormonal method of birth control (such as condoms, spermicidal foam or gel) until a replacement package can be obtained and contact your health care provider for medical advice.
- Do not stop taking birth control pills as this will increase the likelihood of pregnancy. For questions or concerns about birth control products, talk to a health care professional, including about alternatives. Talk to a health care professional if you have any questions or concerns about your birth control product.
- Read the Consumer Information Leaflet included in the ALYSENA™ packaging for detailed instructions in case of a missed dose.
- Pharmacists are reminded to:
- Check each blister pack of ALYSENA™ 21 and ALYSENA™ 28 before dispensing it to make sure the pills look as they should.
- Report any unusual pills to the company (Apotex Inc) and to Health Canada.
REPORT HEALTH OR SAFETY CONCERNS
Managing marketed health product-related side effects depends on health care professionals and consumers reporting them. Any case of unexpected side effects in patients receiving ALYSENA™28 and ALYSENA™ 21 should be reported to Apotex Inc. or Health Canada.
APOTEX INC. CONTACT INFORMATION
150 Signet Drive
Toronto, ON
M9L 1T9
Telephone: To report a suspected adverse reaction associated with the use of ALYSENA™ 28 and ALYSENA™ 21 (Levonorgestrel and Ethinyl Estradiol tablets, USP 100 mcg/ 20 mcg) patients may contact Apotex Inc. by calling 1-800-667-4708 (select prompt 2), by email at [email protected] or by fax at 1-866-429-9133 or 416-401-3819.
To correct your mailing address or fax number, contact Apotex Inc.
You can report any suspected adverse reactions associated with the use of health products to Health Canada by:
- Calling toll-free at 1-866-234-2345; or
- Visiting MedEffect Canada's Web page on Adverse Reaction Reporting (http://www.hc-sc.gc.ca/dhp-mps/medeff/report-declaration/index-eng.php) for information on how to report online, by mail or by fax.
Yours Sincerely,
Original signed by
Colin D'Cunha MBBS,MHSc,FRCPC
Director, Global Medical Affairs
Apotex Inc
SOURCE Apotex Inc.

Share this article