Presented at the British Thoracic Society December 2018.
LONDON, ON, Dec. 12, 2018 /CNW/ - When spacer chambers were introduced in the 1980s, they were a game changer in asthma treatment, overcoming the challenges faced by users with poor inhaler technique. Over the years, their popularity has proliferated, with a wide range of lower-priced chambers available worldwide. Although they may appear interchangeable, they can differ significantly in terms of drug delivery.
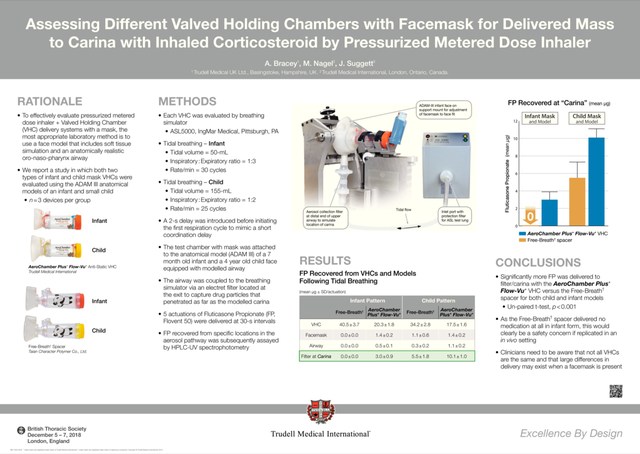
This was borne out in a recent in vitro study presented at the British Thoracic Society Annual Winter Meeting.1 Researchers evaluated the gold standard AeroChamber Plus* Flow-Vu* chamber with a lower-cost chamber, the Free-Breath device, simulating use in both infants and children. Both chambers have a mask and are suitable for children from 1 to 5 years old. When comparing the potential drug delivery into children's lungs (using a Ventolin MDI), the Free-Breath device delivered only 55 percent of the drug as compared to the AeroChamber Plus* Flow-Vu * chamber. Most significantly, results showed that the Free-Breath infant device—which is intended for use by children under the age of 18 months—delivered no drug at all.
"This study was a real eye-opener for me," said Dr. Will Carroll, a Respiratory Paediatrician from Stoke-on-Trent. "I had assumed that if a device was available for prescription, that studies of effectiveness would have been undertaken."
Global asthma guidelines recommend using a spacer chamber that has documented efficacy in young people.2
Using a spacer chamber is critical for young people to overcome obstacles to the drug making its way to the lungs.
The aerosol contents of an HFA inhaler are under pressure and released quickly, making it difficult to coordinate the inhalation of particles. As a result, much of the delivered drug is often deposited to the back of the throat and is then swallowed, increasing the risk of side effects. Spacer chambers are designed to hold the small drug particles in the chamber until the patient is ready to inhale, thus reducing the need for good coordination between inhalation and inhaler actuation.2,3 For infants and young children, coordinating inhalation with actuation of the puffer is just too difficult; using a spacer chamber allows a child to breath normally while inhaling the medication through the puffer.
Spacer chamber design can make a difference to delivery.
For example, leakages between the facemask and the face can affect inhalation. The AeroChamber Plus* Flow-Vu* chamber has a unique feedback feature that helps provide visual assurance of a good facemask seal. The Flow-Vu* inhalation indicator moves with inhalation and allows caregivers to count the number of breaths taken.
Bottom line? Focusing only on a lower price could result in the selection of a spacer chamber that can compromise efficacy.
"A study like this could have important safety implications," Dr. Carroll stressed. "The key message for doctors and allied health professionals prescribing spacer devices is 'prescriber beware.'"
About the AeroChamber Plus* Flow-Vu* chamber
The AeroChamber* brand is a trusted brand with global recognition in the field of respiratory devices, with safety and efficacy validated in numerous third party clinical evaluations amongst various patient populations. It is the chamber most recommended by leading MDI pharmaceutical companies.4 https://www.trudellmed.com
* trade-marks and registered trade-marks of Trudell Medical International © TMI 2018. All rights reserved.
REFERENCES |
|
1. |
Bracey A, Suggett J, Nagel M. Assessing different valved holding chambers with facemask for delivered mass to carina with inhaled corticosteroid by pressurized metered-dose inhaler. Presented at The British Thoracic Society Winter Meeting, December 5-7, 2018. |
2. |
Global initiative for asthma (GINA) guidelines 2018. Global strategy for asthma management and prevention. |
3. |
Lavorini F, Fontana GA. Targeting drugs to the airways: the role of spacer devices. Expert Opin Drug Deliv 2009;6(1):91-102. |
4. |
AeroChamber brand of holding chambers. Study Summary (September 2018). Available from: https://www.trudellmed.com/aerochamber-study-summary |
SOURCE Trudell Medical International

For clinical inquiries, please contact: Jason Suggett, PhD, BPharm, MBA, Group Director, Science and Technology, Trudell Medical International, Tel.: +1 519 455 7060

Share this article