Public Advisory - Magnets in certain Philips Respironics sleep therapy masks may cause serious injury to some individuals Français
OTTAWA, ON, Sept. 26, 2022 /CNW Telbec/ -
Summary

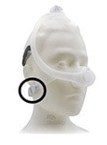

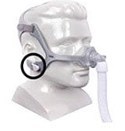
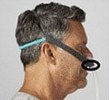
- Product: Philips Respironics sleep therapy masks with magnets in the headgear clips or straps (Amara View Full Face Mask; DreamWisp Nasal Mask; DreamWear Full Face Mask; Wisp and Wisp Youth Nasal Mask; and Therapy Mask 3100 NC/SP)
- Issue: Health products – New safety information
- What to do: STOP USING the affected mask if you or any of your household members, caregivers or bed partners have any of the implanted devices listed below in the "What you should do" section that may be affected by magnets. Obtain another mask that has no magnets. Keep implanted metallic medical devices or objects NOT listed below that may be affected by magnetic fields at least 6 inches—or approximately 15 cm—away from the magnet, and either continue using or seek a replacement mask that has no magnets.
Affected products
Product |
Amara View Full Face Mask |
DreamWisp Nasal Mask |
DreamWear Full Face Mask |
Wisp and Wisp Youth Nasal Mask |
Therapy Mask 3100 NC/SP |
Issue
Certain Philips Respironics sleep therapy masks used with Continuous Positive Airway Pressure (CPAP) or Bi-Level Positive Airway Pressure (BiPAP) machines have magnets in their headgear clips or straps. These magnets could cause safety concerns for people who may be vulnerable to magnetic fields.
The sleep therapy mask magnets are used to attach the headgear clips or straps to the mask. Their magnetic field can potentially affect their function or displace some metallic medical devices or objects (e.g., pacemakers, metallic stents, metal in the eye) which may result in serious injury.
Philips Respironics is adding a contraindication and is updating product warnings and instructions for use (IFU) to help prevent injury to patients and those close to them, who may be vulnerable to magnetic fields.
Philips Respironics has received international reports of potential magnet interference with patient medical devices. These reports include: pacemaker alarms and interference, arrhythmia (irregular heart beat), pacemaker failure leading to replacement, need for shunt adjustment, resetting of automatic implantable cardioverter defibrillator, seizures, defibrillator shutting off periodically and cognitive issues. To date, no incidents have been reported in Canada.
Health Canada is advising patients that the affected masks should not be used by patients if they or a person in close proximity to them including, household members, caregivers, or bed partners have any of the implanted metallic medical devices or objects listed below inside their bodies. For patients who have other metallic medical devices or objects inside their bodies that are not included on the list below but that may be affected by magnets, the manufacturer warns that the affected masks should be kept at least 6 inches (approximately 15 cm) away from the device/object.
Please note this issue is not related to the recall of Philips Respironics CPAP and BiLevel PAP machines and mechanical ventilators due to issues involving the sound abatement foam, which Health Canada first communicated about in June 2021 and provided an update to in July 2022.
What you should do:
STOP USING the affected mask if you or any of your household members, caregivers or bed partners have implanted metallic medical devices or objects that may be affected by magnets, including but not limited to:
- Pacemakers
- Implantable cardioverter defibrillators (ICD)
- Neurostimulators
- Magnetic metallic implants/electrodes/valves placed in upper limbs, torso, or higher (neck and head)
- CSF (cerebral spinal fluid) shunts (e.g., VP [ventriculo peritoneal] shunt)
- Aneurysm clips
- Embolic coils
- Intracranial aneurysm intravascular flow disruption devices
- Metallic cranial plates, screws, burr hole covers, and bone substitute devices
- Metallic splinters in the eye
- Ocular implants (e.g., glaucoma implants, retinal implants)
- Certain contact lenses with metal
- Implants to restore hearing or balance that have an implanted magnet (such as cochlear implants, implanted bone conduction hearing devices, and auditory brainstem implants)
- Magnetic denture attachments
- Metallic gastrointestinal clips
- Metallic stents (e.g., aneurysm, coronary, tracheobronchial, biliary)
- Implantable ports and pumps (e.g., insulin pumps)
- Hypoglossal Nerve Stimulators
- Devices labelled as MR (magnetic resonance) unsafe
- Magnetic metallic implants not labelled for MR or not evaluated for safety in a magnetic field
Ask your physician or health care provider to help you find another mask without magnets that you can use with your device. You can also contact Philips Respironics customer service at 1-800-345-6443 or the Durable Medical Equipment (DME) provider that supplied you with the affected mask for more information on non-magnetic mask options.
Once you have a new mask, properly dispose of the mask that has magnets.
If you or any of your household members, caregivers or bed partners have implanted metallic medical devices or objects that can be affected by magnetic fields but that are NOT on the list above:
Ensure the mask is kept at least 6 inches (approximately 15 cm) away from any implanted metallic medical devices or objects that may be affected by magnetic fields. You can continue using your mask, or you can follow the steps outlined above to replace it.
For people without metallic devices or objects in their bodies:
You can keep using your mask if you, your household members, caregivers or bed partners do not have implanted metallic medical devices or objects in the body.
Report any health product-related adverse reactions or complaints to Health Canada.
Additional information
For more information, including device model numbers, visit Recall Notice: Respironics Masks (2022-09-16).
Alert / recall type: Public Advisory
Category: Health products – Medical Devices
Published by: Health Canada
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, 613-957-2983, [email protected]; Public Enquiries: 613-957-2991, 1-866 225-0709

Share this article