Public Advisory - Recall of one lot of pms-Hydromorphone due to packaging error that could potentially lead to overdose Français
OTTAWA, ON, Aug. 20, 2022 /CNW/ -

- Product: pms-Hydromorphone, 2 mg tablets (DIN 00885436) marketed by Pharmascience Inc. Sold to pharmacies in bottles of 100 tablets for dispensing to consumers.
- Issue: Health product – product safety
- What to do: Check your bottle of pms-Hydromorphone to ensure you have the correct tablets. Stop using product from the affected lot and contact your pharmacy immediately for a replacement product.
- Who this is for: General public and pharmacists
- 2 mg: Round, biconvex, orange tablet debossed and half-scored with "2"on one side of the tablet and "pms" on the other side.
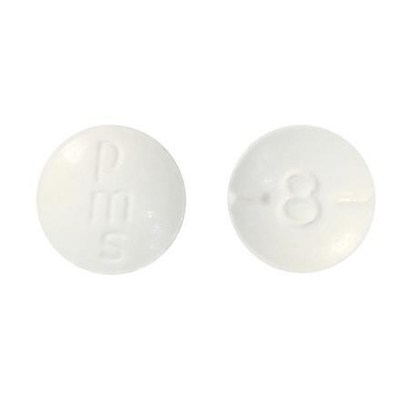
- 8 mg: Round, biconvex, white tablet debossed and half-scored with "8"on one side of the tablet and "pms" on the other side.
Product |
DIN |
Lot |
Expiry date |
Pharmascience Inc. pms-Hydromorphone Bottles of 100 tablets |
00885436 |
639268 |
31-01-2026 |
Pharmascience Inc. is recalling one lot of pms-Hydromorphone, 2 mg tablets, (lot 639268) as the bottles may contain hydromorphone tablets of a different strength (8 mg), meaning they contain higher amounts of hydromorphone. Products from the affected lot were sold between May 2022 and August 2022.
The 2 mg hydromorphone tablets are round and orange with a "2" on one side of the tablet and "pms" on the other side. The 8 mg hydromorphone tablets are round and white with an "8" on one side of the tablet and "pms" on the other side.
Hydromorphone is an opioid medication used to treat pain and opioid use disorder.
Accidentally taking too much hydromorphone (one dose of 8 mg of hydromorphone instead of one dose of 2 mg) or suddenly increasing the dose of hydromorphone could potentially lead to an overdose, which can be life-threatening even in people who have some tolerance to opioids.
Symptoms of hydromorphone overdose may include: dizziness, drowsiness, confusion, slow heartbeat, slow and difficult breathing, cold and clammy skin, seizure, coma and death.
Patients and their caregivers should be aware of the signs and symptoms of hydromorphone overdose, and should seek medical attention immediately if any of these symptoms are noted. If naloxone is available, be prepared to administer it.
The Department is monitoring the company's recall and will inform the public if any new health risks are identified.
- Check your bottle of pms-Hydromorphone to ensure you have the correct tablets. The 2 mg hydromorphone tablets are round and orange with a "2" on one side of the tablet and "pms" on the other side. The 8 mg hydromorphone tablets are round and white with an "8" on one side of the tablet and "pms" on the other side.
- Stop using product from the affected lot and contact your pharmacy immediately for a replacement product.
- If you have accidentally taken the 8 mg hydromorphone tablet instead of the 2 mg hydromorphone tablet and you have any health concerns, or are unsure whether you have taken too much hydromorphone, contact your healthcare professional, hospital emergency department or regional poison control centre immediately, even if there are no symptoms. If naloxone is available, be prepared to administer it.
- If you have any questions about the recall, contact Pharmascience Inc. at [email protected] or 1-888-550-6060.
- Report any health product adverse events or complaints to Health Canada.
Alert / recall type: Public Advisory
Category: Drugs
Companies: If you have any questions about the recall, contact Pharmascience Inc. at [email protected] or 1-888-550-6060.
Published by: Health Canada
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, 613-957-2983, [email protected]; Public Enquiries: 613-957-2991, 1-866 225-0709

Share this article