Public advisory - Unauthorized health products seized from stores in the Greater Toronto Area because they may pose serious health risks Français
OTTAWA, ON, Aug. 23, 2023 /CNW/ -
Summary


- Product: Unauthorized health products labelled to contain a prescription drug
- Issue: Health products – Unauthorized product, Product safety
- What to do: Do not use these products. Buy your prescription drugs only from licensed pharmacies. Return them to your local pharmacy for proper disposal. Consult a health care professional if you have used any of these products and have health concerns.
Product
|
Prescription drug on the label |
Retail location |
Date added |
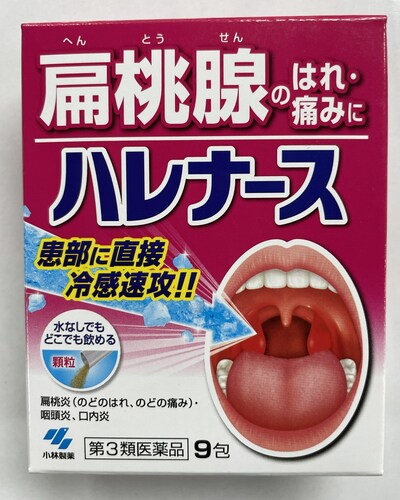
Kobayashi Harenasu (Granules for swallowing, for tonsil or throat inflammation) |
Labelled to contain tranexamic acid |
L'Amour Beauty Locations:
670 Yonge St, Toronto, ON
505 Hwy 7 #37, Thornhill, ON |
2023-08-22 |
Prevalin α ointment (Skin treatment) |
Labelled to contain prednisolone valerate acetate |
Asia Food Mart 2150 McNicoll Ave Scarborough, ON |
2023-08-22 |
Rohto antimicrobial eye drops (Eye drops) |
Labelled to contain aminocaproic acid |
L'Amour Beauty 670 Yonge St, Toronto, ON |
2023-08-22 |
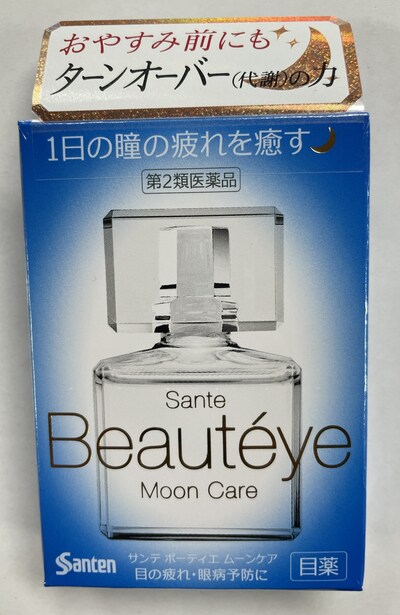
Santen Sante Beauteye Moon Care (Eye drops) |
Labelled to contain aminocaproic acid |
Asia Food Mart 2150 McNicoll Ave Scarborough, ON
L'Amour Beauty Locations:
670 Yonge St, Toronto, ON
505 Hwy 7 #37, Thornhill, ON
5365 Yonge St, North York, ON |
2023-08-22 |
Santen Sante Beauteye (Eye drops) |
Labelled to contain neostigmine methylsulfate |
Asia Food Mart 2150 McNicoll Ave Scarborough, ON |
2023-08-22 |
Santen Sante Beauteye Contact (Eye drops) |
Labelled to contain neostigmine methylsulfate |
Asia Food Mart 2150 McNicoll Ave Scarborough, ON
L'Amour Beauty Locations:
670 Yonge St, Toronto, ON
505 Hwy 7 #37, Thornhill, ON
5365 Yonge St, North York, ON |
2023-08-22 |
Santen Sante Fx V+ (Eye drops) |
Labelled to contain aminocaproic acid |
Asia Food Mart 2150 McNicoll Ave Scarborough, ON |
2023-08-22 |
Health Canada is warning consumers about four additional unauthorized health products, including eye drops, a skin ointment and granules for swallowing (to relieve tonsil or throat inflammation), that it seized from Asia Food Mart and three L'Amour Beauty locations in the Greater Toronto Area. The products are labelled to contain prescription drugs and may pose serious health risks.
Original Advisory (July 31, 2023): Unauthorized Santen brand eye drops seized from stores in the Greater Toronto Area because they may pose serious health risks
Health Canada is warning consumers about unauthorized Santen brand eye drops that it seized from Asia Food Mart and three L'Amour Beauty locations in the Greater Toronto Area, Ontario. The products are labelled to contain prescription drugs and may pose serious health risks.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, efficacy and quality and may pose a range of serious health risks. For example, they could contain high-risk ingredients, such as prescription drugs, additives or contaminants that may or may not be listed on the label. These ingredients could interact with other medications and foods. In addition, these products may not actually contain the active ingredients that consumers would expect them to contain to help maintain and improve their health.
Prescription drugs should be used only under the supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects. Prescription drugs can only be legally sold to consumers in Canada with a prescription.
- Do not use these products. Return the product to your local pharmacy for proper disposal.
- Consult a health care professional if you have used any of these products and have health concerns.
- Buy your prescription drugs only from licensed pharmacies.
- Buy only authorized health products. Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related side effects or complaints to Health Canada.
Aminocaproic acid: This is a prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Neostigmine methylsulfate: There are no approved eye drops containing neostigmine methylsulfate on the Canadian market. In the past, drugs similar to neostigmine were used to treat glaucoma. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts, allergic reactions, iris cysts, retinal detachment and the potential for causing a specific type of glaucoma attack. In addition, absorption into the nose via the tear duct may cause serious cardiac and respiratory side effects.
Prednisolone valerate acetate is a prescription corticosteroid drug available in Canada as eye drops used to treat inflammation of several parts of the eye. It has not been approved for use in creams in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), changes in skin colour, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, and swelling. Prednisolone acetate should not be used in patients who are allergic to prednisolone acetate or to any ingredient in the formulation. Prednisolone acetate is not to be used in children and is not recommended for use during pregnancy or breastfeeding.
Tranexamic acid is a prescription drug used to prevent or reduce bleeding by slowing the breakdown of blood clots. The drug is usually administered by mouth or intravenously. Tranexamic acid should be used with caution with blood thinners and anti-inflammatories, estrogen therapy, medicines used to help blood clotting, birth control pills, hydrochlorothiazide, desmopressin, ranitidine, or nitroglycerin.
Également disponible en français
SOURCE Health Canada

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709, [email protected]

Share this article