Public advisory - Unauthorized health products seized from online retailer "UU Zone" may pose serious health risks Français
UPDATE: March 13, 2024: Additional unauthorized health products seized from online retailer "UU Zone" may pose serious health risks.
OTTAWA, ON, March 13, 2024 /CNW/ - Further to its communication on October 25, 2023, Health Canada is warning consumers about additional unauthorized health products seized from the online retailer UU Zone, based in Markham, ON. These products are labelled to contain prescription, controlled, or over-the-counter drugs, and may pose serious health risks.
The list of products seized has been added to the Affected Products table below. Information on the health risks associated with these drugs as well as information for consumers can be found further below.











October 25, 2023: Unauthorized health products seized from online retailer "UU Zone" may pose serious health risks
Summary
- Product: Unauthorized health products labelled to contain drugs
- Issue: Health products – Product safety; Unauthorized product
- What to do: Do not use these products. Return products to your local pharmacy for proper disposal. Consult a healthcare professional if you have used any of these products and have health concerns. Read product labels to verify that health products have been authorized for sale by Health Canada.
Affected products
Product |
Promoted use |
Drug on the label |
Date added |
Acnes25
|
Acne skin treatment |
Labelled to contain ibuprofen piconol 3% |
October 25, 2023 |
Borraginol A |
Hemorrhoidal ointment |
Labelled to contain prednisolone acetate |
October 25, 2023 |
Daiichi Sankyo Healthcare Makiron Patch
|
Anti-itch patch |
Labelled to contain dexamethasone |
October 25, 2023 |
PAIR Acne Cream
|
Acne skin treatment |
Labelled to contain ibuprofen piconol 3% |
October 25, 2023 |
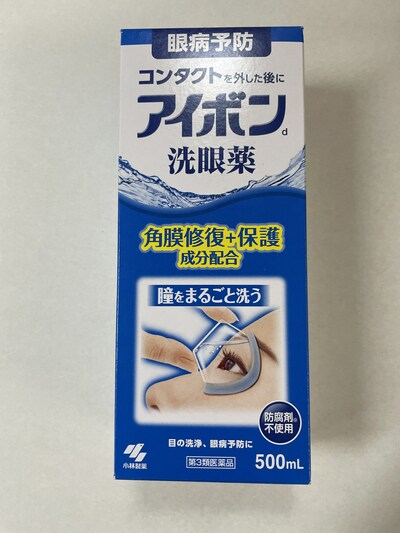
Rohto Digieye anti blue light eye drops (clear) |
Eye drops |
Labelled to contain neostigmine methylate |
October 25, 2023 |
Rohto Digieye anti blue light eye drops (yellow) |
Eye drops |
Labelled to contain neostigmine methylate |
October 25, 2023 |
Rohto Z! eye drops |
Eye drops |
Labelled to contain neostigmine methylate |
October 25, 2023 |
Taisho Canker Sore Patch/Stomatitis patch Taisho Quick Care |
Canker patch for children 5 years old and up |
Labelled to contain triamcinolone acetonide |
October 25, 2023 |
Rohto Z! Pro |
Eye drops |
Labelled to contain neostigmine methylsulfate |
March 13, 2024 |
Sante Beauteye Moon Care |
Eye drops |
Labelled to contain aminocaproic acid |
March 13, 2024 |
Sante PC Contact |
Eye drops |
Labelled to contain neostigmine methylsulfate |
March 13, 2024 |
Kobayashi Eyewash (Green/Turquiose)) |
Eye drops |
Labelled to contain aminocaproic acid |
March 13, 2024 |
Sante 40 Plus |
Eye drops |
Labelled to contain neostigmine methylsulfate and aminocaproic acid |
March 13, 2024 |
Kobayashi AL Eyewash (Green) |
Eye drops |
Labelled to contain aminocaproic acid |
March 13, 2024 |
Kobayashi Eyewash (Blue) |
Eye drops |
Labelled to contain aminocaproic acid |
March 13, 2024 |
Sante PC |
Eye drops |
Labelled to contain neostigmine methylsulfate |
March 13, 2024 |
Rohto Koukin Antibacterial Eyedrop |
Eye drops |
Labelled to contain aminocaproic acid |
March 13, 2024 |
Sante Beauteye Contact |
Eye drops |
Labelled to contain neostigmine methylsulfate |
March 13, 2024 |
Taisho Pabron Gold A Granules Cold Medication |
Cold medication |
Labelled to contain dihydrocodeine phosphate and acetaminophen |
March 13, 2024 |
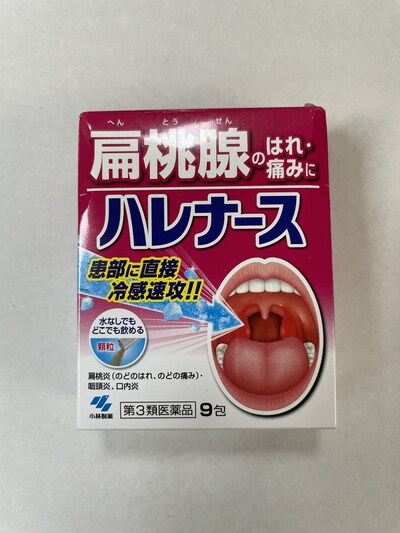
Kobayashi Harenasu Medicine for Throat Pain and Swelling |
Cold medication |
Labelled to contain tranexamic acid |
March 13, 2024 |
Health Canada is warning consumers about unauthorized health products it seized from UU Zone, an online retailer, based in Markham, ON. The products are labelled to contain medicinal drugs and may pose serious health risks.
Selling unauthorized health products in Canada is illegal. Unauthorized health products have not been approved by Health Canada, which means that they have not been assessed for safety, efficacy and quality and may pose a range of serious health risks. For example, they could contain high-risk ingredients, such as prescription drugs, additives or contaminants that may or may not be listed on the label. These ingredients could interact with other medications and foods. In addition, these products may not actually contain the active ingredients that consumers would expect them to contain to help maintain and improve their health.
Prescription drugs should only be used under the advice and supervision of a health care professional because they are used to treat specific conditions and may cause serious side effects. Prescription drugs can only be legally sold to consumers in Canada with a prescription.
What you should do
- Do not use these products. Return the products to your local pharmacy for proper disposal.
- Consult a health care professional if you have used any of these products and have health concerns.
- Buy your prescription drugs only from licensed pharmacies.
- Buy only authorized health products. Read product labels to verify that health products have been authorized for sale by Health Canada. Authorized health products have an eight-digit Drug Identification Number (DIN), Natural Product Number (NPN) or Homeopathic Drug Number (DIN-HM). You can also check whether products have been authorized for sale by searching Health Canada's Drug Product Database and Licensed Natural Health Product Database.
- Report any health product-related side effects or complaints to Health Canada.
Background
Aminocaproic acid is a prescription drug ingredient used to decrease bleeding in various clinical situations. Exposure to aminocaproic acid in the eye may affect the eye itself, and the acid may be absorbed through the tear ducts into the blood. Side effects may include watery eyes, vision changes, headache, dizziness, nausea, muscle weakness and skin rash.
Dexamethasone is a prescription corticosteroid drug available in Canada in various formulations and is used to treat inflammatory conditions. It has not been approved for use in creams in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), changes in skin colour, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, and swelling. Dexamethasone should not be used by people who are allergic to dexamethasone or to any ingredient in the formulation, people who have systemic fungal infections, or people who have received live virus vaccines. Dexamethasone is generally not recommended for use during pregnancy.
Dihydrocodeine phosphate is a controlled substance regulated under the Controlled Drugs and Substances Act and is similar to the opioid codeine. Although codeine is approved in Canada, Health Canada has not authorized any drug products containing dihydrocodeine. Dihydrocodeine tablets are approved in certain countries for the relief of severe and chronic pain or as cough suppressants. Common adverse reactions include dizziness, headache, vertigo, visual disturbances, confusion, euphoria, nausea, and constipation. As with all opioids, use of dihydrocodeine may lead to drug dependence (addiction). Dihydrocodeine may cause slowed or stopped breathing, which can lead to severe drowsiness, unconsciousness, and death (opioid overdose). People with other medical conditions, including but not limited to those that affect the lungs, liver or kidney, may be at a higher risk of overdose. Children are more susceptible to overdose due to their smaller size. Dihydrocodeine should not be used by those who are pregnant or breastfeeding, or by those with an allergy to dihydrocodeine. Using dihydrocodeine with other central nervous system depressants (e.g., alcohol, sleeping pills, etc.) may worsen its effects. In cases of overdose, naloxone can temporarily reverse the effects of dihydrocodeine.
Ibuprofen piconol 3% is a topical (applied to the skin) non-steroidal anti-inflammatory drug (NSAID) used to relieve burns. Health Canada has not approved any drugs containing ibuprofen for topical use. Ibuprofen absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time, or on damaged skin. Topical ibuprofen may cause serious side effects in people who are allergic to ibuprofen, aspirin or other NSAIDs, or who are asthmatic. Use of topical ibuprofen may also cause serious side effects such as stomach and intestinal bleeding, renal (kidney) dysfunction or failure, or cardiovascular dysfunction or failure in people who have problems with these organs. Topical ibuprofen can also cause serious side effects in people who are pregnant or breastfeeding, such as delayed and increased duration of labour.
Neostigmine methylsulfate is a prescription drug available in Canada as an injection used to prevent and treat urine retention and intestinal complications after surgery, to reverse the paralyzing effect of certain drugs used in surgery and in shock therapy, and to control symptoms of myasthenia gravis (a disease that causes weakness in the skeletal muscles). It has not been approved for use as eye drops in Canada. In the past, drugs similar to neostigmine were used to treat glaucoma, a group of eye diseases traditionally characterized by elevated pressure within the eye. These medications are no longer widely used because of the significant number of potential eye-related side effects, including blurred distance vision, frontal headaches, twitching lids, red eyes, cataracts (clouding of the normally clear lens of the eye), allergic reactions, iris cysts, retinal detachment with symptoms of reduced vision, the sudden appearance of flashes of light or floaters that could lead to permanent vision loss, and the potential for causing a specific type of glaucoma attack with potentially permanent vision loss. In addition, absorption into the nose via the tear duct may cause serious cardiac and respiratory side effects.
Prednisolone acetate is a prescription corticosteroid drug available in Canada as eye drops used to treat inflammation of several parts of the eye. It has not been approved for use in creams or ointments in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), changes in skin colour, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, and swelling. Prednisolone acetate should not be used in patients who are allergic to prednisolone acetate or to any ingredient in the formulation. Prednisolone acetate is not to be used in children and is not recommended for use during pregnancy or breastfeeding.
Tranexamic acid is a prescription drug used to prevent or reduce bleeding by slowing the breakdown of blood clots. The drug is usually administered by mouth or intravenously. Tranexamic acid should be used with caution with blood thinners and anti-inflammatories, estrogen therapy, medicines used to help blood clotting, birth control pills, hydrochlorothiazide, desmopressin, ranitidine, or nitroglycerin.
Triamcinolone acetonide is a prescription corticosteroid drug available in Canada as a topical cream, ointment and dental paste, and is used to treat inflammatory conditions. Triamcinolone acetonide has not been approved for use as a patch in Canada. Common side effects for topical corticosteroids include skin atrophy (thin and fragile skin with reduced elasticity), skin blood vessel changes (e.g., spider veins), changes in skin colour, stretch marks, swelling, dry skin, burning sensation, local irritation, rash, redness, itching, thinning hair or excessive hair growth, infections and allergic reactions. Topical corticosteroids absorbed through the skin or through the mouth may cause side effects throughout the body, especially when used over a large surface area and for a long time. This risk is greater in children, who may absorb proportionally larger amounts and be more susceptible to side effects. Systemic side effects could include high blood pressure, high blood sugar, blurred vision, uneven heartbeats, weakness, swelling, suppression of the adrenal glands and worsening of stomach ulcer. Triamcinolone acetonide should not be used by people who are allergic to triamcinolone acetate or to any ingredient in the formulation, people with untreated bacterial or fungal infections involving the skin, people with fungal, viral or bacterial infections of the mouth or throat, people with tuberculosis, stomach ulcer or diabetes mellitus, and people with certain viral diseases such as herpes simplex or chicken pox. Use of triamcinolone acetonide as a dental paste should be stopped if a local irritation develops. Triamcinolone acetonide is not recommended for use during pregnancy or breastfeeding.
Également disponible en français
SOURCE Health Canada (HC)

Media Enquiries: Health Canada, (613) 957-2983, [email protected]; Public Enquiries: (613) 957-2991, 1-866 225-0709, [email protected]
Share this article